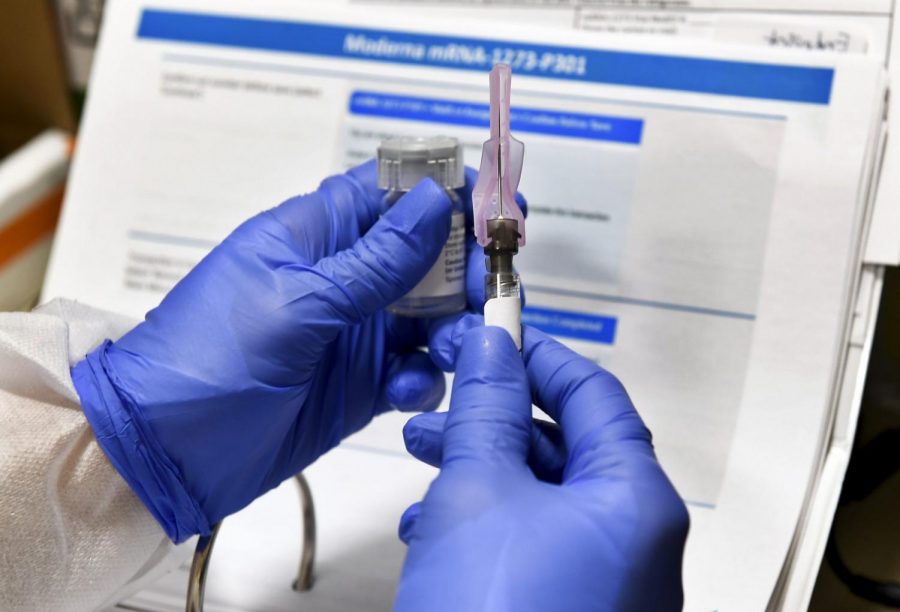
Moderna asking US, European regulators to OK its virus shots
Associated Press
• November 30, 2020
Load More Stories
TRENDING
WEEKLY PRINT ISSUE
NEWSLETTER SIGN-UP






